Answer:
V = 1.95 L.
Step-by-step explanation:
Hell there!
In this case, according to the following reaction between sodium metal and water:
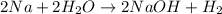
We can realize that the moles of hydrogen can be calculated by using the initial mass of sodium, its atomic mass (23.0 g/mol) and the 2:1 mole ratio of sodium to hydrogen to obtain:
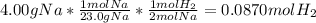
Finally, we calculate the volume of hydrogen by using the ideal gas equation whereas the pressure is 1 atm and the temperature 273.15 K according to the STP conditions:

Regards!