Answer: 3.024 g grams of hydrogen are needed to convert 76 grams of chromium(III) oxide,
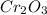
Step-by-step explanation:
The reaction equation for given reaction is as follows.
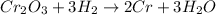
Here, 1 mole of
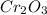 reacts with 3 moles of
reacts with 3 moles of
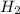 .
.
As mass of chromium (III) oxide is given as 76 g and molar mass of chromium (III) oxide
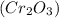 is 152 g/mol.
is 152 g/mol.
Number of moles is the mass of substance divided by its molar mass. So, moles of
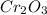 is calculated as follows.
is calculated as follows.
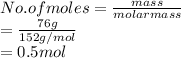
Now, moles of
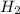 .given by 0.5 mol of
.given by 0.5 mol of
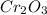 is calculated as follows.
is calculated as follows.
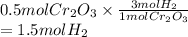
As molar mass of
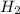 is 2.016 g/mol. Therefore, mass of
is 2.016 g/mol. Therefore, mass of
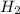 is calculated as follows.
is calculated as follows.
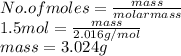
Thus, we can conclude that 3.024 g grams of hydrogen are needed to convert 76 grams of chromium(III) oxide,
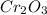 .
.