Answer:
288 g/mol
Step-by-step explanation:
What is diffusion?
• It is a process whereby the random motion of particles move from a region of higher concentration to a region of lower concentration.
Graham's law of diffusion of gas
• states that at constant conditions of temperature and pressure, the rate of diffusion of gases is inversely proportional to the square root of their molar masses
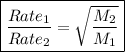
Calculations
Oxygen exist as O₂ at room temperature, thus its molar mass is 2(16)= 32.
Let the rate of O₂ be Rate₁, while the rate and molar mass of the unknown gas be Rate₂ and M₂ respectively.
Since O₂ diffuses 3 times as fast as the unknown gas,
Rate₁= 3(Rate₂)
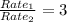
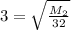
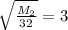
Square both sides:
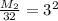
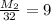
Multiply both sides by 32:
M₂= 9(32)
M₂= 288