Answer:
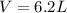
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to realize that we need to use the ideal gas equation in order to calculate the volume of CO2 but firstly calculating the moles:
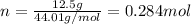
Then, we proceed with the ideal gas equation to solve for volume:

Regards!