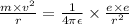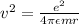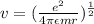Answer:
Step-by-step explanation:
The net force on electron is electrostatic force between electron and proton in the nucleus .
Fc =
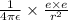
This provides the centripetal force for the circular path of electron around the nucleus .
Centripetal force required =
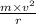
So