Answer:
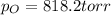
Step-by-step explanation:
Hello there!
In this case, according to the given equation, it is possible to calculate the mass of oxygen by using the Dalton's law, considering that the total pressure is 850 torr and the vapor pressure of water at 30°C is 31.8 torr:
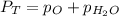
Thus, we solve for the pressure of oxygen as follows:
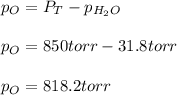
Best regards!