Answer:

Step-by-step explanation:
1 mole of any substance contains the same number of particles. The particles can vary (atoms, molecules, formula units), but there are always 6.022*10²³ particles. In this case, the particles are formula units of potassium nitrate or KNO₃.
Let's create a ratio.
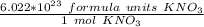
Since we are trying to find the formula units in 0.250 moles, we multiply by that number.
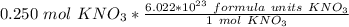
The units of moles of potassium nitrate cancel.
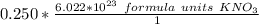
The denominator of 1 can be ignored, so we can make a simple multiplication problem.
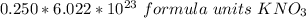
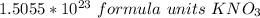
If we round to the nearest tenth, the 0 in the hundredth place tells us to leave the 5 in the tenth place.
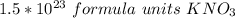
0.250 moles of potassium nitrate is approximately equal to 1.5*10²³ formula units of potassium nitrate and choice B is correct.