Answer: It completely dissociates in water is a characteristic of strong acid.
Step-by-step explanation:
An acid which dissociates completely to give hydrogen ions
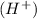 is called a strong acid.
is called a strong acid.
For example, HCl is a strong acid and it dissociates completely as follows.
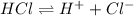
Strong acids are able to conduct electricity in water as more number of ions are present in the solution as compared to the ions present in a solution of weak acid.
Strong acids increase the concentration of
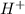 ions.
ions.
Thus, we can conclude that it completely dissociates in water is a characteristic of strong acid.