Answer: There is 842.54 grams of sodium carbonate are produced when 5.3 moles of sodium phosphate reacts with aluminum carbonate.
Step-by-step explanation:
Chemical equation depicting reaction between sodium phosphate and aluminum carbonate is as follows.
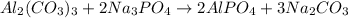
As this equation contains same number of atoms on both reactant and product side. So, this equation is a balanced equation.
According to the equation, 2 moles of sodium phosphate is giving 3 moles of sodium carbonate.
Therefore, sodium carbonate formed by 5.3 moles of sodium phosphate is as follows.
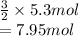
As number of moles is the mass of substance divided by its molar mass. So, mass of sodium carbonate ( molar mass = 105.98 g/mol) is as follows.
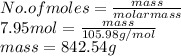
Thus, we can conclude that there is 842.54 grams of sodium carbonate are produced when 5.3 moles of sodium phosphate reacts with aluminum carbonate.