Answer:
The concentration of the unknown HCl is 0.0851 M.
Step-by-step explanation:
The equation of neutralization:
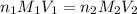
Where:
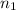 = Basicity of acid
= Basicity of acid
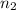 = Acidity of base
= Acidity of base
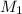 = concentration of acid
= concentration of acid
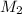 = concentration of base
= concentration of base
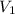 = Volume of acid used in neutralization
= Volume of acid used in neutralization
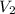 = Volume of base used in neutralization
= Volume of base used in neutralization
We have:
The acidity of HCl =
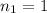
The concentration of HCl solution used =
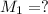
The volume of HCl used in titration =
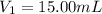
The acidity of NaOH =
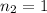
The concentration of NaOH solution used =
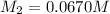
The volume of NaOH used in titration =
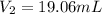
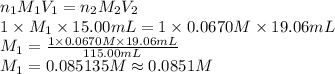
The concentration of the unknown HCl is 0.0851 M.