Answer:
The gas with molar mass 83.9 g is Krypton, Kr
Step-by-step explanation:
We know that as per Ghram's law of diffusion,
r'/ r= square root (M/M')
0r
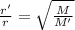
Where
r= rate of diffusion of Cl2
r'= rate of diffusion of unknown gas= 0.920r
Also, M= molar mass of Cl2= 71 g/ mol
And, M'= molar mass of unknown gas which we have to find
Let us substitute the values in above equation
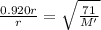
solving we get M' = 83.9 g/mol
Therefore, the gas with molar mass 83.9 g is Krypton,Kr