Answer: The statement 'a molecule or ion that donates the hydrogen in a hydrogen bond is a hydrogen bond donor' is true.
Step-by-step explanation:
A molecule or ion that donates hydrogen in a hydrogen bond is called a hydrogen donor.
For example,
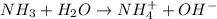
Here,
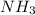 is donating the hydrogen ion to water molecule. Whereas water is accepting the hydrogen ion so water is a hydrogen bond acceptor. Therefore, hydrogen bond is formed by
is donating the hydrogen ion to water molecule. Whereas water is accepting the hydrogen ion so water is a hydrogen bond acceptor. Therefore, hydrogen bond is formed by
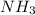 .
.
Thus, we can conclude that the statement 'a molecule or ion that donates the hydrogen in a hydrogen bond is a hydrogen bond donor' is true.