The question is: 10 g of carbonic acid H2CO3 are dissolved in 150 g of water. Determine the% m / m concentration of that solution?
Answer: The% m / m concentration of that solution is 6.66%.
Step-by-step explanation:
Given: Mass of solute = 10 g
Mass of solvent = 150 g
Formula used to calculate the %m/m is as follows.
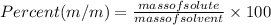
Substitute the values into above formula as follows.
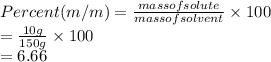
Thus, we can conclude that the% m / m concentration of that solution is 6.66%.