Answer: There are
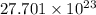 molecules in 4.6 moles of ammonium nitrate
molecules in 4.6 moles of ammonium nitrate
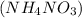 .
.
Step-by-step explanation:
According to the mole concept, there are
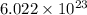 molecules present in 1 mole of every substance.
molecules present in 1 mole of every substance.
Hence, the number of molecules present in 4.6 moles of ammonium nitrate are calculated as follows.
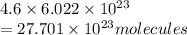
Thus, we can conclude that there are
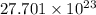 molecules in 4.6 moles of ammonium nitrate
molecules in 4.6 moles of ammonium nitrate
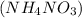 .
.