Answer:
The specific heat of the sample unknown metal is approximately 0.45 J/g °C.
General Formulas and Concepts:
Thermodynamics
Specific Heat Formula:
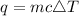
- m is mass (g)
- c is specific heat capacity (J/g °C)
- ΔT is the change in temperature
Step-by-step explanation:
Step 1: Define
Identify variables.
m = 112 g
ΔT = 20.0 °C
q = 1004 J
Step 2: Solve for c
- Substitute in variables [Specific Heat Formula]:
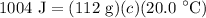
- Simplify:
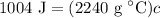
- Isolate c:
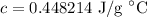
- Round [Sig Figs]:
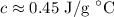
∴ specific heat capacity c is equal to around 0.45 J/g °C.
---
Topic: AP Chemistry
Unit: Thermodynamics