Answer: The value of equilibrium constant, K, is
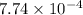 .
.
Step-by-step explanation:
The reaction equation is as follows.

Now, the expression for equilibrium constant of this reaction is as follows.
![K = ([SO_(2)]^(2)[O_(2)])/([SO_(3)]^(2))](https://img.qammunity.org/2022/formulas/chemistry/high-school/e02g0ot4wixioauttt693h243vfm5zysqb.png)
Substitute the given values into above expression as follows.
![K = ([SO_(2)]^(2)[O_(2)])/([SO_(3)]^(2))\\= (((0.210)/(15.4))^(2) * ((0.354)/(15.4)))/(((8.51 * 10^(-2))/(15.4)))\\= 7.74 * 10^(-4)](https://img.qammunity.org/2022/formulas/chemistry/high-school/tvzykyhib1w62nvkpgw0qntnldjqwyamcu.png)
Thus, we can conclude that the value of equilibrium constant, K, is
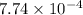 .
.