Step-by-step explanation:
If the density of the object is more than that of water, it will sink. Otherwise it will float. The density of water is 1 g/mL.
Substance 1,
Mass, m = 450 g, Volume, V = 90 mL
Density = mass/volume
So,
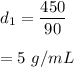
It will sink.
Substance 2,
Mass, m = 35 g, Volume, V = 70 mL
Density = mass/volume
So,
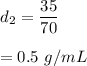
It will float.
Substance 3,
Mass, m = 24 g, Volume, V = 12 mL
Density = mass/volume
So,
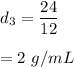
It will sink.