Answer: A reaction which occurs when equivalent quantities of H" (or
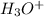 ) and OH" are mixed is a neutralization reaction.
) and OH" are mixed is a neutralization reaction.
Step-by-step explanation:
When acid and base chemically combine together to form sat and water then it is know as neutralization reaction.
An acidic substance gives hydrogen ions
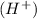 on dissociation and a basic substance gives hydroxide ions
on dissociation and a basic substance gives hydroxide ions
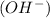 on dissociation.
on dissociation.
For example,
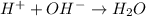 is a neutralization reaction where an acid and base are mixed together to form water.
is a neutralization reaction where an acid and base are mixed together to form water.
No salt is formed as because here the reactants are not present as compounds.
In a hydrolysis reaction, water molecule(s) is added to the reactant molecules.
For example,
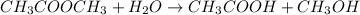
Oxidation is the process of addition of oxygen to a molecule and removal of hydrogen.
Reduction is the process of addition of hydrogen in a compound and the removal of oxygen atom.
Therefore, we can conclude that reaction which occurs when equivalent quantities of H" (or
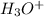 ) and OH" are mixed is a neutralization reaction.
) and OH" are mixed is a neutralization reaction.