Answer: The energy of a photon with a wavelength of 820 nm is
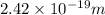 .
.
Step-by-step explanation:
Given : Wavelength = 820 nm
Convert nm into meter as follows.
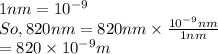
The relation between energy and wavelength is as follows.
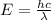
where,
E = energy
h = Planck's constant =
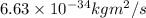
c = speed of light =
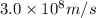
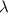 = wavelength
= wavelength
Substitute the values into above formula as follows.
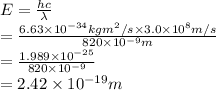
Thus, we can conclude that energy of a photon with a wavelength of 820 nm is
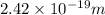 .
.