Answer:
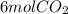
Step-by-step explanation:
Hello there!
In this case, according to the described reaction, we can set up the chemical equation as shown below:
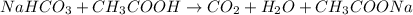
We can evidence the 1:1 mole ratio of baking soda (NaHCO3) to CO2 and therefore the produced moles of latter are calculated as shown below:
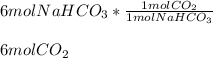
Best regards!