Answer:
Rate of Ar= 1.65 x
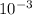 mol/h
mol/h
Step-by-step explanation:
Here we'll use Graham's Law where
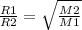
R= rate of effusion and M= molar mass
Let's plug in what we know.
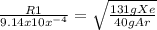
You could switch them where Xe is M1 (on the bottom) instead of M2, but I find it easier when the unknown is R1, where it acts like a whole number. It makes the algebra part of the equation easier.
Let's solve for R1.
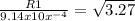
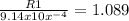
R1= 1.089 x
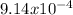
R1= 1.65 x
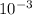 mol/h
mol/h