Answer:
E = 1.38 x 10⁸ eV = 138 MeV
Step-by-step explanation:
The energy associated with the given wavelength can be found from the following formula:
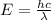
where,
E = Energy of electron = ?
h = Plank's Constant = 6.625 x 10⁻³⁴ J.s
c = Speed of Light = 3 x 10⁸ m/s
λ = wavelength = 9 fm = 9 x 10⁻¹⁵ m
Therefore,
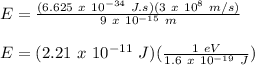
E = 1.38 x 10⁸ eV = 138 MeV