Answer:
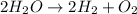
Step-by-step explanation:
Hello there!
In this case, for the reaction by which water is decomposed to molecular hydrogen and oxygen:
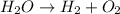
It is necessary to perform the inspection balance process since there is a dissimilar number of atoms of oxygen on both sides; therefore, by putting a 2 on water we balance oxygen:
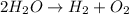
But now, there are four hydrogens on the left; therefore, we put a 2 on hydrogen to finally balance it:
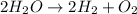
And obviously, the coefficient in oxygen is an unwritten 1.
Best regards!