Answer:
![[Sr^(2+)]=5.66x10^(-4)M](https://img.qammunity.org/2022/formulas/chemistry/high-school/lil9591ax82ptcub3oxxw7w353rw9waojo.png)
Step-by-step explanation:
Hello there!
In this case, given the solubility equilibrium of strontium sulfate:
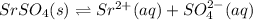
Whose equilibrium expression is:
![Ksp=[Sr^(2+)][SO_4^(2-)]](https://img.qammunity.org/2022/formulas/chemistry/high-school/m3p1e2hvhxm4divsc36gw2hy0bucpmbac3.png)
In such a way, we can introduce the molar solubility, s, in the equation to obtain:
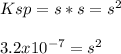
Then, we apply the square root to obtain:
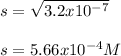
Which is also the concentration of strontium ions:
![[Sr^(2+)]=5.66x10^(-4)M](https://img.qammunity.org/2022/formulas/chemistry/high-school/lil9591ax82ptcub3oxxw7w353rw9waojo.png)
Best regards!