Answer:
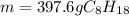
Step-by-step explanation:
Hello there!
In this case, according to the given chemical reaction, we can set up the heat of reaction per mole of gasoline as shown below:
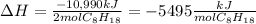
Now, since the total heat is obtained by multiplying the moles and heat of reaction, we can calculate the moles as shown below:
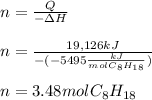
Finally, since the molar mass of gasoline is 114.22 g/mol, we can easily calculate the mass as follows:
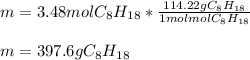
Best regards!