Solution :
a). In this experiments, we can determine the hardness of the water or
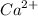 ion concentration in the sample.
ion concentration in the sample.
b). The Possible errors are :
- During the weighing of calcium standard preparation, if the balance is not traced properly, it will cause an error.
- By dilution of standard from the stock will lead a dilution error of 0.1 mL for 100 mL.
- Misreading the liter values will give an error.
c). Depending upon the calcium content present in the soil, we conclude that the fertile percent of the soil as well as the mineral capacity of the soil.