Answer:
a. 650 moles of S
b. 60 grams of FeS₂
Step-by-step explanation:
Fe + 2S → FeS₂
from the mole ratio 1 mole of S yields
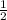 mole of FeS₂
mole of FeS₂
a. ∴ 650 moles of S is required to produce 325 moles of FeS₂
b. 0.5 mole of S yields 0.25 mole of FeS₂
molar mass of FeS₂ = 120gmol⁻¹
mass of FeS₂ = 120gmol⁻¹ ₓ 0.25mol
= 60grams