Given :
A sample of unknown gas has a temperature of 378 K.
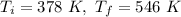
When the temperature is increased to 546 K, the volume also increases to 1047 ml.
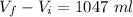
Solution :
We know, temperature is directly proportional to volume.
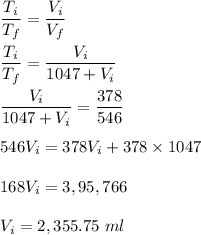
Therefore, the original volume is 2.355.75 ml.