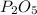Answer: The empirical formula is
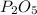
Step-by-step explanation:
Mass of O = 5.717 g
Mass of P= (10.150-5.717) g = 4.433 g
Step 1 : convert given masses into moles.
Moles of O =
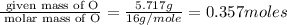
Moles of P=
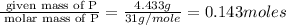
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For O =
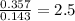
For P =
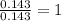
The ratio is to be converted to whole number
Thus whole number ratio of O : P =
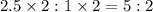
Hence the empirical formula is