Answer: The volume of nitrogen gas that is required is 13944 L.
Step-by-step explanation:
Given values:
Number of molecules of ammonia gas =
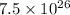
According to the mole concept:
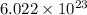 number of molecules are present in 1 mole of a compound
number of molecules are present in 1 mole of a compound
So,
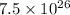 number of molecules will be present in
number of molecules will be present in
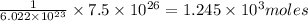 of ammonia gas
of ammonia gas
The chemical equation for the formation of ammonia gas follows:
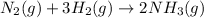
According to the stoichiometry of the reaction:
2 moles of ammonia gas are produced from 1 mole of nitrogen gas
So,
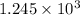 moles of ammonia gas will be produced from
moles of ammonia gas will be produced from
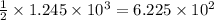 moles of nitrogen gas
moles of nitrogen gas
At STP conditions:
1 mole of a gas occupies 22.4 L of volume
So,
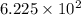 moles of nitrogen gas will occupy
moles of nitrogen gas will occupy
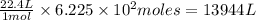 of volume
of volume
Hence, the volume of nitrogen gas that is required is 13944 L.