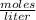Answer:
The molarity of the resulting NaHSO₄ solution is 0.05
Step-by-step explanation:
Molarity or Molar Concentration is the number of moles of solute that are dissolved in a certain volume.
The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution:
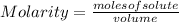
Molarity is expressed in units
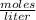 .
.
Molar mass is the amount of mass that a substance contains in one mole. Being the molar mass of the compound NaHSO₄ equal to 120.1 g/mole, then the number of moles that 12 grams contain is calculated by:
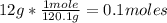
And being 2 L the volume of the solution, then replacing in the definition of molarity you get:
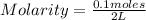
Molarity= 0.05
The molarity of the resulting NaHSO₄ solution is 0.05