Answer: The hydrogen ion concentration in molarity is 0.013
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pOH is calculated by taking negative logarithm of hydroxide ion concentration and pH is calculated by taking negative logarithm of hydrogen ion concentration
Putting in the values:
![pOH=-\log[7.609* 10^(-13)]](https://img.qammunity.org/2022/formulas/chemistry/college/6v31u6qz7arp44dbdxr4gczyvxb3n0p2dg.png)
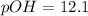
Now ,
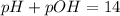
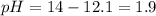
![pH=-\log [H^+]](https://img.qammunity.org/2022/formulas/chemistry/college/d4u8c7rky5aqengst85apsbxbk128yl4er.png)
![[H^+]=0.013M](https://img.qammunity.org/2022/formulas/chemistry/college/7twtj8s3y4j9lkyyc5th344jdpoyfu3jze.png)
The hydrogen ion concentration in molarity is 0.013