Answer: The value of the equilibrium constant Kp for this reaction is

Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as K
For the given balanced chemical reaction:
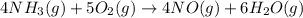
The expression for
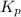 is written as:
is written as:
![K_p=([p_(NO)]^4* [p_(H_2O)]^6)/([p_(NH_3)]^4* [p_(O_2)]^5)](https://img.qammunity.org/2022/formulas/chemistry/college/en7llbpzhc7k3j0euwk5895l37yi22hqbg.png)
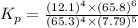
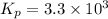
The value of the equilibrium constant Kp for this reaction is
