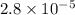Answer: The value of the equilibrium constant for this reaction is
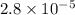
Step-by-step explanation:
To calculate the moles, we use the equation:
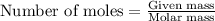
Moles of
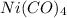 =
=
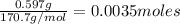
Moles of
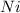 =
=
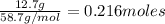
Moles of
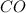 =
=
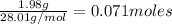
Volume of solution = 2.7 L
Equilibrium concentration of
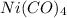 =
=
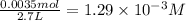
Equilibrium concentration of
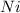 =
=
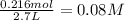
Equilibrium concentration of
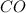 =
=
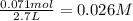
The given balanced equilibrium reaction is,
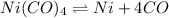
The expression for equilibrium constant for this reaction will be,
![K_c=([Ni]^1* [CO]^4)/([Ni(CO_4]^1)](https://img.qammunity.org/2022/formulas/chemistry/college/jg6mr85l6hrks3h54tbed3zt4wo520h9w7.png)
Now put all the given values in this expression, we get :
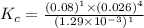
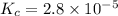
Thus the value of the equilibrium constant for this reaction is