Answer:
Step-by-step explanation:
The left electrode will be positive on the grounds that focus on the concentration that the cell electron moves from a lower concentration fixation to a higher concentration. Thus right electrode will go about and act as an anode and will be negative. Also, the left electrode will be the cathode and will be positive.
The concentration cell
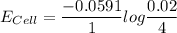
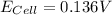
= 136 mV