Answer:
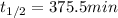
Step-by-step explanation:
Hello!
In this case, since this problem refers to two different temperatures, it is possible to compute the rate constant at 652 K given the half-life at such temperature:
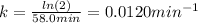
Next, by using the T-variable version of the Arrhenius equation, we can compute the rate constant at 623 K:
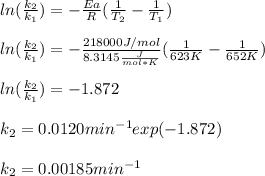
Finally, the half-life at 623 K turns out to be:
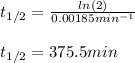
Best regards!