Answer:
C: 0.78 V
Step-by-step explanation:
The Cr2O7 cell has a higher reduction potential, so it will be reduced (as it is a better oxidizing agent). This means that Fe will be oxidized. Its oxidation potential is simply the negative of its reduction potential, 0.45:
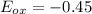 . We know that cell potential is the sum of the reduction and oxidation potentials of each reaction (it is not an extensive property like enthalpy or entropy, so we don't need to worry about multiplying anything). Therefore,
. We know that cell potential is the sum of the reduction and oxidation potentials of each reaction (it is not an extensive property like enthalpy or entropy, so we don't need to worry about multiplying anything). Therefore,
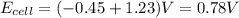 .
.