Answer:
47.6 days
Explanation:
Given formula:
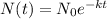
where:
- N₀ = initial mass (at time t = 0)
- N = mass (at time t)
- k = a positive constant
- t = time (in days)
First, find the decay constant (k) for chromium-51.
Given its half-life is about 28 days:
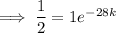
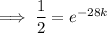
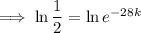
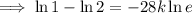
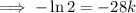
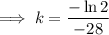
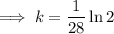
To determine how long it will take for a 650 mg of a sample of chromium-51 to decay to 200 mg, substitute the following values into the formula and solve for t:
- N₀ = 650 mg
- N = 200 mg
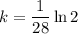
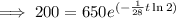
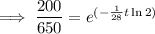
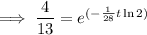
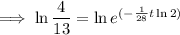
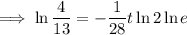
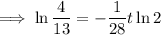
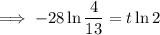
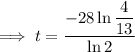
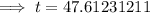
Therefore, it will take 47.6 days (nearest tenth) for a 650 mg of a sample of chromium-51 to decay to 200 mg.