Answer: The pH of the solution is 8.8
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.pOH is calculated by taking negative logarithm of hydroxide ion concentration
![pH=-\log [H^+]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/college/79qpm3uwz8hayr1qtyvcw8.png)
![pOH=-log[OH^-]](https://img.qammunity.org/qa-images/2022/formulas/chemistry/high-school/160evsnfb6wifju9312ajm.png)
pH+pOH=14
Putting in the values:
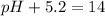
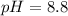
Thus pH of the solution is 8.8