Answer:
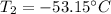
Step-by-step explanation:
Hello there!
In this case, in agreement to the Charles' law which states that the volume and temperature relate to each other via a directly proportional relationship at constant pressure, we can write:
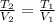
Thus, given V1, V2 and T1 (310 in Kelvin), we can solve for the final temperature as shown below:
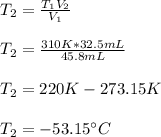
Best regards!