Answer:
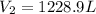
Step-by-step explanation:
Hello there!
In this case, given the pressure, temperature and volume of the gas, we notice that we need the combined ideal gas as shown below:
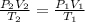
Thus, solving for the final volume, V2, we would obtain:
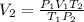
Now, we plug in the data and make sure the temperature must be in Kelvins to obtain:
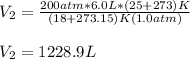
Best regards!