Answer:
Ag2CrO4
silver chromate, 331.966 g/mol
Ag=65%, Cr=15% O=19%
CH4
Methane, 16 g/mol
C=75% H=25%
Ca3(PO4)2
calcium Phosphate, 310 g/mol
Ca=38.7% P=20% O=41.3%
C7H5N3O6
227 g/mol
C=37% H=2.2% N=18.5% O=42.3%
K2C6H5O7
BAIDAR citron, 267 g/mol
CoCl2
Cobalt (ll) Chloride, 129.93
Step-by-step explanation:
Ag2CrO4
Ag=108, Cr=51.996, O=16
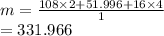
Ag percentage in Ag2CrO4
[(108x2)/331.966]x100
=65%
Cr percentage in Ag2CrO4
[51.966/331.966]x100
=15%
O percentage in Ag2CrO4
[(16x4)/331.966]x100
=19%
CH4
C=12 H=1
12+1x4=16 g/mol
C percentage in CH4
(12/16)x100
75%
H percentage in CH4
(4/16)x100
25%
Ca3(PO4)2
Ca=40 P=31 O=16
40x3+31x2+16x8
=310 g/mol
Ca % in Ca3(PO4)2
[(40x3) /310]x100
38.7%
P % in Ca3(PO4)2
[(31x2)/310]x100
20%
O % in Ca3(PO4)2
[(16x8)/310]x100
41.3%
C7H5N3O6
C=12 H=1 N=14 O=16
12x7+1x5+14x3+16x6
227 g/mol
C % in C7H5N3O6
[(12x7)/227]x100
37%
H % in C7H5N3O6
[(1x5)/227]x100
2.2%
N % in C7H5N3O6
[(14x3)/227]x100
18.5%
O % in C7H5N3O6
[(16x6)/227]
42.3%
- Do the same for BAIDAR cirton and Cobalt (||) Chloride
- percentage composition = [(mass of the elements x no. of elements) /molar mass of the molecule]x 100
please add this on brainleist it took me long time to write