Answer:
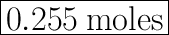
Step-by-step explanation:
To find the number of moles of a solution given it's volume and concentration/molarity, we use the formula;
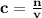
where
- c is the concentration/molarity in M or mol/l.
- n is the number of moles.
- v is the volume in L or dm³.
Since we're finding the number of moles, we make n the subject
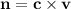
From the question;
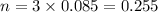
We have the final answer as
0.255 moles