Answer:
Step-by-step explanation:
From the information given:
Mass of BiPO₄ = 0.3451 g
Number of moles of BiPO₄ =
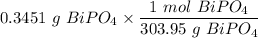

The number of moles of Bi³⁺ in 20.00 mL is:
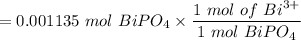
= 0.001135 mol of Bi³⁺
The number of moles of Bi³⁺ in 100 mL stock solution
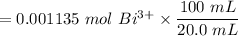
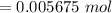
Mass of BSS in 4.9993 g tablets
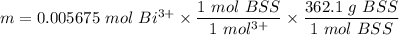
m = 2.055 g BSS
Mass of BSS in 5.0103 g (5 tables)
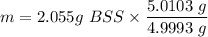
= 2.06 g
∴
The mass of BSS per tablet is
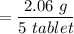
= 0.412 g BSS/ tablet