Answer:
1.59%
Step-by-step explanation:
First, we convert 0.1606 g of AgCl into moles, using its molar mass:
- 0.1606 g ÷ 143.32 g/mol = 1.12x10⁻³ mol AgCl
Then we convert the calculated moles of Cl (equal to AgCl moles) into moles of DDT:
- 1.12x10⁻³ mol Cl *
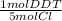 = 2.24x10⁻⁴ mol DDT
= 2.24x10⁻⁴ mol DDT
Now we convert 2.24x10⁻⁴ moles of DDT into grams, using its molar mass:
- 2.24x10⁻⁴ mol DDT * 354.48 g/mol = 0.0794 g
Finally we calculate the percentage of DDT in the sample:
- 0.0794 g / 5.0 * 100% = 1.59%