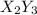The given question is incomplete. the complete question is:
The electron arrangement ions
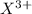 and
and
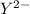 are 2, 8, and 2, 8, 8 respectively.
are 2, 8, and 2, 8, 8 respectively.
Write the electronic arrangement of the elements X and Y.
Write the formula of the compound that would be formed between X and Y.
Answer: 1. The electronic arrangement for X and Y are 2,8, 3 and 2,8,5 respectively.
The formula of the compound formed is
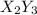
Step-by-step explanation:
Electronic configuration is the arangement of electrons in an atom in order of increasing energies.
Cations are formed when electrons are lost by atoms and anions are formed when electrons are gained by atoms.
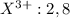
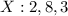
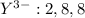
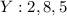
For formation of a neutral ionic compound, the charges on cation and anion must be balanced.
Here element X is having an oxidation state of +3 called as
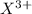 cation and
cation and
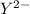 is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral
is an anion with oxidation state of -2. Thus they combine and their oxidation states are exchanged and written in simplest whole number ratios to give neutral