Answer:
Vanadium
Step-by-step explanation:
When an atom gains electrons, they fill different orbitals sets according to a specific order. You can follow the periodic table in increasing order. This electron ends with
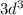 so you just look at the 3rd element in the d-block. This is vanadium.
so you just look at the 3rd element in the d-block. This is vanadium.