Answer:
Nonpolar
Step-by-step explanation:
Well,
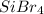 or better known as silicon tetrabromide, is considered nonpolar.
or better known as silicon tetrabromide, is considered nonpolar.
To start,
Polar covalent bonds are a common component of polar molecules, which are covalent chemical compounds. Due to an uneven distribution of bonding valence electrons caused by varying electronegativity values in the connecting atoms, these bonds have partial charge separation. A dipole moment that is directed in a certain direction is represented by each polar covalent link. According to VSEPR theory, the complex itself has a certain molecular geometry at the center atom. Because of this geometry and the polar covalent bonds, the compound itself may occasionally exhibit a net dipole moment.
To put this simply,
Because bromine is more electronegative than silicon, each covalent link between Si and Br is polar. The shape of the molecule, however, prevents it from being polar. In other words, its geometry.
Here is some information of the SiBr4 Lewis Structure, Geometry, Hybridization, and Polarity.
Tetrabromosilane, often known as silicon tetrabromide, is an inorganic molecule in which one Si atom and four Br atoms are covalently connected. It is produced using silicon and bromine vapors at temperatures over 600 °C. SiBr4 is a colorless liquid with an extremely oppressive smell. Its capacity to hydrolyze and emit HBr gas, which has an unpleasant odor, as demonstrated in the equation, is the reason of this distinctive stench.
SiBr4 + 2H2O → SiO2 + 4 HBr
Thanks.