Answer: The vapor pressure of the solution at
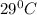 is 29.86 mm Hg
is 29.86 mm Hg
Step-by-step explanation:
As the relative lowering of vapor pressure is directly proportional to the amount of dissolved solute.
The formula for relative lowering of vapor pressure will be,
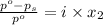
where,
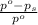 = relative lowering in vapor pressure
= relative lowering in vapor pressure
i = Van'T Hoff factor = 1 (for non electrolytes)
 = mole fraction of solute
= mole fraction of solute
=
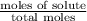
Given : 9 moles of
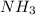 are dissolved in 50 L or 50000 ml of water
are dissolved in 50 L or 50000 ml of water
mass of water =
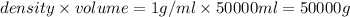
moles of solvent (water) =
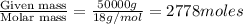
Total moles = moles of solute + moles of solvent = 9 mol + 2778 mol = 2787
 = mole fraction of solute
= mole fraction of solute
=
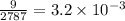
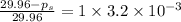
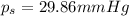
Thus the vapor pressure of the solution at
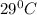 is 29.86 mm Hg
is 29.86 mm Hg