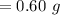Answer:
"0.60 g" is the appropriate solution.
Step-by-step explanation:
The given values are:
Volume of base,
= 30 ml
Molarity of base,
= 0.05 m
Molar mass of acid,
= 400 g/mol
As we know,
⇒
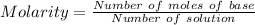
On substituting the values, we get
⇒
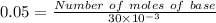
⇒
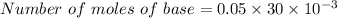
⇒
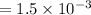
hence,
⇒
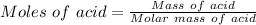
On substituting the values, we get
⇒
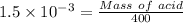
⇒
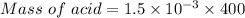
⇒